Application Example
Equilibrium Simulations of the Basic Oxygen Furnace Process
Two elements that are very important in steelmaking and steel refining are sulfur (S) and phosphorus (P). Both elements are usually undesirable and must be removed from the liquid steel. This is generally done by transferring them to a CaO-rich slag phase. In this example, we show how the Process Metallurgy Module in Thermo-Calc can be used to investigate suitable conditions to remove P from liquid steel using a Basic Oxygen Furnace (BOF). In a later example, the Module is used to investigate desulphurization in a ladle furnace.
The example can also help you determine optimal operation conditions and predict and optimize costs of raw materials and recycling.
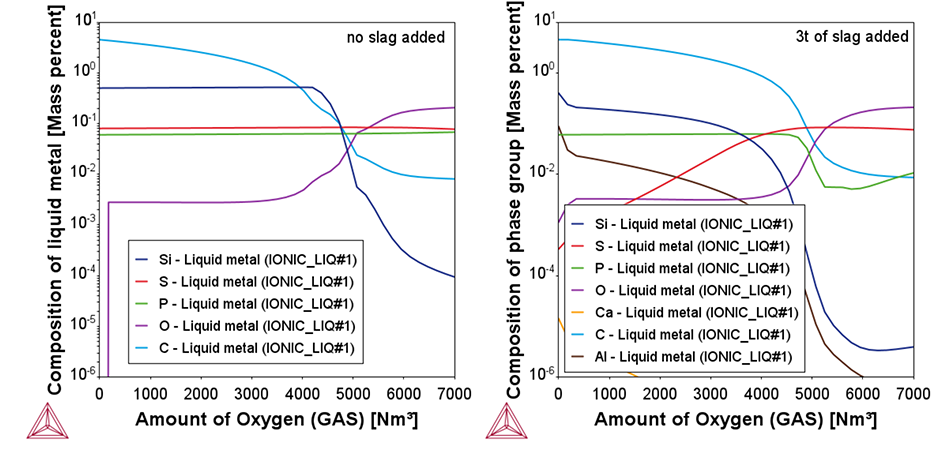 Two plots from the example showing the change of the chemical composition of the liquid metal as a function of added oxygen. In the left plot, no slag formers are added. In the right plot, 3t of CaO rich slag formers are added, leading to a reduction in P content during the end of the blow, as shown by the green line.
Two plots from the example showing the change of the chemical composition of the liquid metal as a function of added oxygen. In the left plot, no slag formers are added. In the right plot, 3t of CaO rich slag formers are added, leading to a reduction in P content during the end of the blow, as shown by the green line.
Thermo-Calc Software offers two application examples showing how the Process Metallurgy Module can be used to calculate the Basic Oxygen Furnace Process. This example uses equilibrium calculations to gain a general understanding of your BOF process. The other example gives detailed instructions on how to simulate the kinetics of the Basic Oxygen Furnace Process.
View the Kinetics Version of the Example