Application Example
Desulphurization in a Ladle Furnace
The ladle furnace (LF) fulfils many purposes in the steelmaking process. Desulphurization, which we will focus on in this example, is merely one of them.
Desulphurization is usually performed by transferring sulfur (S) that is dissolved in the liquid metal to a CaO-rich slag phase, as shown in the image:
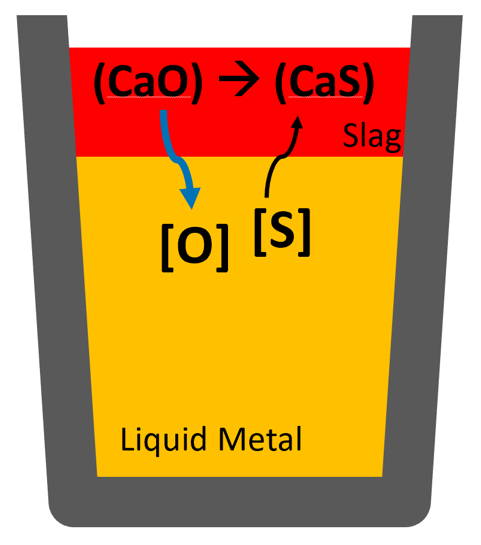
The principle of desulphurization is to transfer the sulfur dissolved in the liquid steel to a CaO-rich slag phase. This process is typically carried out in a ladle furnace (LF).
For this process to be successful, two conditions need to be fulfilled:
- The slag must be fully liquid (liquid fraction > 0.9). This is required for kinetic reasons. The slag phase must be fluid so that it can emulsify with the liquid steel and form a large surface area where the reaction between steel and slag can take place.
- The slag must take up a large amount of S from the liquid steel (have a “high sulfur capacity”) so that a significant amount of sulfur will move from the liquid steel to the slag phase.
About the Example
The example demonstrates how the Process Metallurgy Module in Thermo-Calc can be used to explore these two conditions for an equilibrium between liquid steel and slag in a ladle furnace, trying to find good slag compositions. We also investigate why excessive slag carry-over from the BOF or EAF is detrimental for desulphurization in the LF and who how slag conditioning with CaC2 as fluxing agent can recover the desulphurizing ability of the slag.